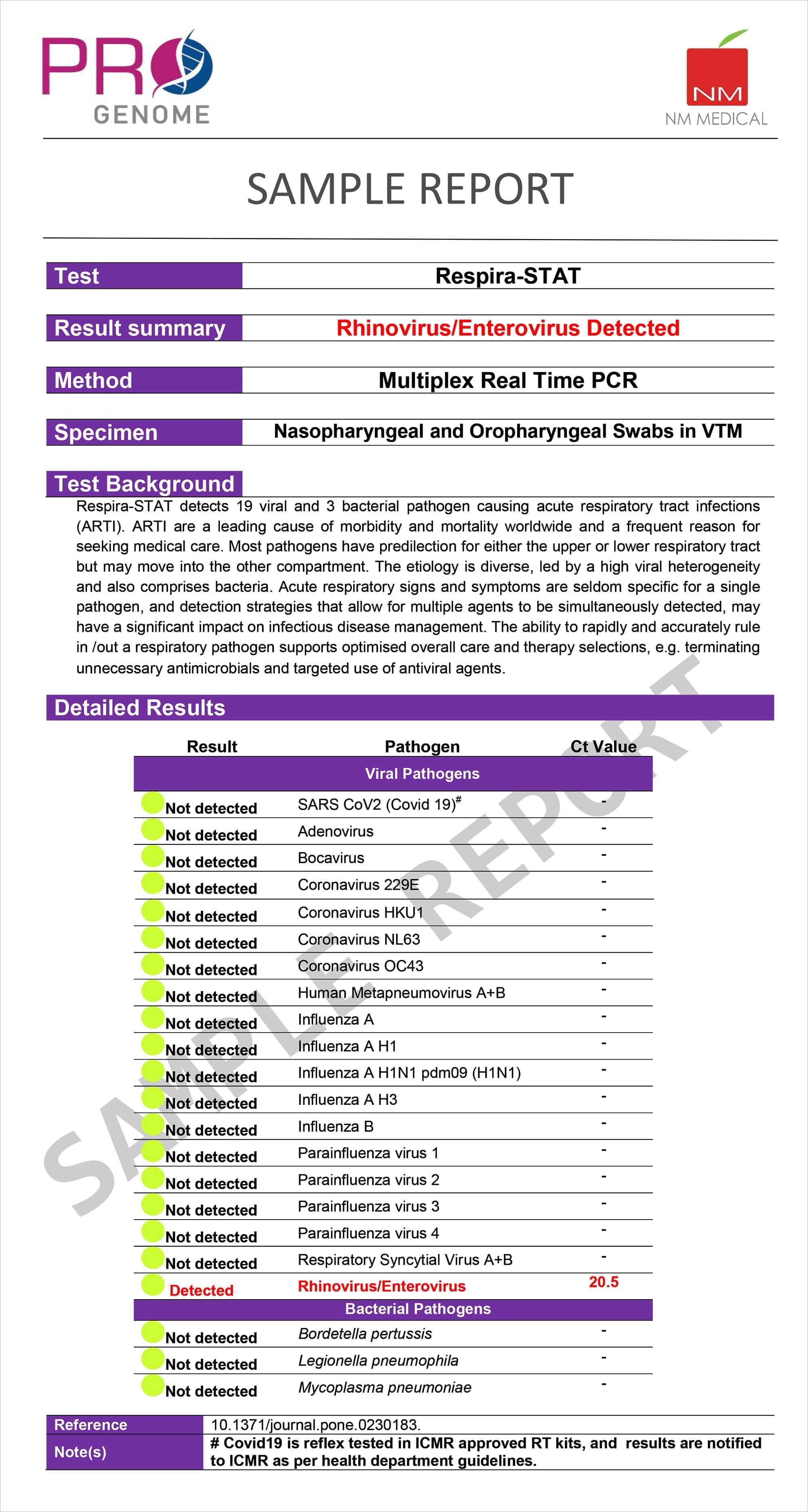
Multiplex Real Time PCR panel for a faster, more sensitive and cost-effective Infection Identification and Management
Detects 22 respiratory targets including 2019-nCov (SARS-CoV-2), from Nasopharyngal swab (NPS)
- Utilizes real-time PCR to deliver results with CT values
- Faster Turn Around Time
- Emergency Use Authorization (EUA) for SARS CoV2 testing by US-FDA
Pathogens
- SARS CoV2 (Covid 19)
- Adenovirus
- Bocavirus
- Coronavirus 229E / HKU1/ NL63/ 0C43
- Human Metapneumovirus A+B
- Influenza A/ H1N1 pdm09 (H1N1) / H3 / B
- Parainfluenza virus 1 / 2 / 3 / 4
- Respiratory Syncytial Virus A+B
- Rhinovirus/Enterovirus
- Bordetella pertussis
- Legionella pneumophila
- Mycoplasma pneumoniae
Sample Report